By Dr. Bill Barnett, D.V.M.
The focus on human and animal nutrition during the past five decades has been to determine the level of micronutrients necessary to prevent deficiency diseases and to define the nutrient and energy intake that would promote maximum growth and reproductive health. In layman’s terms, what’s defined as a healthy level of food consumption? Additionally, how many calories should my dog eat?
A Nutritional Paradigm for the 21st Century
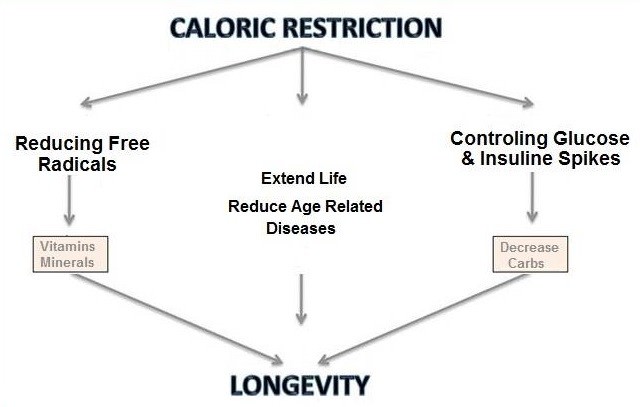
Enter, a new era of Optimum Nutrition. That is, the ideal levels of micronutrients, macronutrients and total energy needed during all developmental and maintenance phases over a complete lifetime. Nutrient levels that will promote optimum health, minimize disease, and maximize life span.
This noble endeavor has great implications for the health and well-being of men, women and children around the globe, and will have a comparable impact on the health and longevity of our companion animals should we choose to adapt these principles.
One primary area of nutritional research concerns total energy intake and its impact on health and longevity. It is quite obvious that over-nutrition can lead to obesity with its accompanying health risks. Generally, energy intake in the adult animal sufficient to maintain weight without accumulation of excess body fat, has been thought to be ideal. However, a recent body of research now suggests that slight under-nutrition may, in fact, be an integral component of optimal nutrition.
Well-controlled studies in a diverse number of animal species have shown that calorie restriction over a lifetime creates significant positive beneficial changes in physiological functions accompanied by a decrease in age-related disorders. The latest studies in dogs, primates, and humans also indicate that modest underfeeding over periods of months to years results in similar physiological changes and suggest that extended calorie control may also affect various pathologies associated with aging. Underfed animals exhibit increased vigor and energy, maintain youthful mental and physiological functions well beyond normal fed control animals, and remain relatively disease free. Well, how many calories should my dog eat? Taken collectively, these results provide an impressive case for under-nutrition without malnutrition as an important component of long-term health for animals.
Calorie Restriction and Longevity
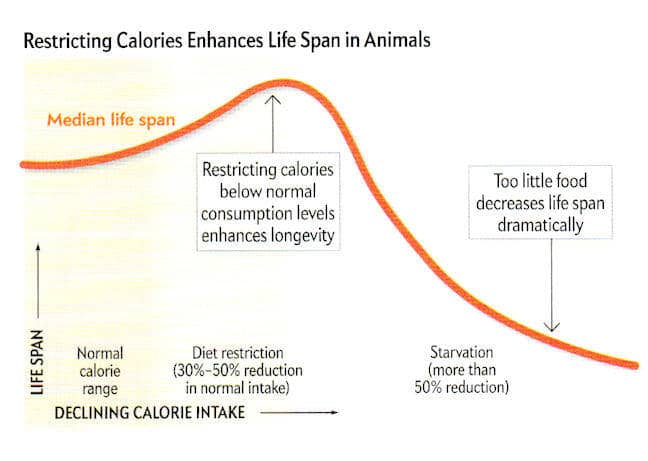
How many calories should my dog eat? Can restricting calorie intake increase the life span of animals? Early studies in the 1930’s by McCay and his associates suggested this was true. McCay allowed a group of rats free access to food throughout their lifetime while restricting the daily food intake of a second group. Results were conclusive… the underfed rats were observed to live, on average, twice as long as the control animals.
This intriguing phenomenon has been confirmed by a large number of recent studies. Collectively, results from this body of research make a compelling case; that by reducing food intake the maximum life span of several species can be increased significantly. Even more interesting is the observation that underfed, long-lived animals are free of most age-associated chronic diseases.
A Life Long Canine Study Reveals How to Help your Dog Live Longer
This study was conducted by Ralston Purina, who asked himself the same question, “how many calories should my dog eat?” What it shows is that pet owners have the ability to increase the lifespan of their pet simply by feeding less food.
In the first-ever lifelong canine restriction study, researchers at Purina have proven that a dog’s median life span can be extended by 15 percent (nearly two years for the Labrador Retrievers used in this study) by feeding to ideal body condition through dietary restriction. The findings were published in the Journal of the American Veterinary Medical Association.
The 14 year “Life Span” study found that dogs that consumed 25 percent fewer calories than their litter-mates during their lifetimes maintained a lean or ideal body condition resulting in a longer life. This study provides the most significant data to date on the effects of diet restriction in the dog. According to Dennis Lawler, DVM, Purina scientist and a lead study investigator. “What’s exciting about this study is that, for the first time in a larger mammal, we have shown scientifically that by simply feeding to maintain ideal body condition throughout a dog’s life, we can increase length of life while delaying the visible signs of aging.”
Study Design to “How Many Calories Should My Dog Eat?”

All dogs were fed the same 100 percent nutritionally complete and balanced diets (puppy, then adult) for the entire period of the study, from eight weeks of age until death – only the quantity was different.
Dogs were weighed weekly as puppies, periodically as adolescents and then weekly as adults. Beginning at six years of age, they were evaluated annually for ideal body condition. Other health indicators, body fat mass, lean body mass, bone mass, glucose and insulin levels along with cholesterol and triglyceride levels were measured to assess their overall condition and health.
Study Results to “How Many Calories Should My Dog Eat?”
Study findings revealed that the median life span of the lean-fed dogs was extended by 15 percent or nearly two years. Median life span (the age at which 50 percent of dogs in the group died) was 11.2 years for the control group versus 13 years for the lean-fed dogs.
By age 10, only three lean-fed dogs had died, compared to seven control group dogs. At the end of the twelfth year, 11 lean-fed dogs were alive with only one control dog surviving. Twenty-five percent of the lean-fed group survived to 13.5 years, while none of the control group dogs lived to 13.5 years.
The study showed that the lean-fed dogs maintained a significantly leaner body condition from 6 to 12 years of age than the control group dogs. On average, the lean-fed group weighed less, had lower body fat, and after a certain age, experienced a two-year delay in the loss of lean body mass as they aged, compared to the control group dogs.
In addition, according to observations of the researchers, the control dogs exhibited more visible signs of aging, such as graying muzzles, impaired gaits and reduced activity at an earlier age than the lean-fed dogs.
The study provides some insight into human health as well.
Dr. Richard Weindruch, University of Wisconsin professor of medicine and expert in the diet restriction field said, “This study is significant for human as well as canine health because it’s the first study completed in a larger mammal that proves the significant power that diet restriction wields in extending life and delaying the markers of aging. From this study, we can extrapolate that large mammals, including humans, can potentially live healthier and longer through diet restriction.” So… how many calories should my dog eat? According to this study, not nearly as much as we expect
What is most impressive about the research on underfeeding and longevity over the last two decades is the variety of disciplines represented by current researchers and the broad scope of physiological and disease processes affected by restricted feeding. Prominent scientists include Roy Walford, MD, Pathology, UCLA; Edward Masoro, Ph.D., Physiology, University of Texas; Robert Good, MD, Ph.D., Immunology, University of South Florida and former head of Memorial Sloan Kettering Cancer Institute; Richard Cutler, Ph.D., Genetics, National Institute of Health; and, William Regelson, MD, Gerontology, University of Virginia.
The effects of food restriction studies are global in nature and include:
- The ability to maintain a broad array of physiological processes in a youthful state.
- Retarding or preventing age-related diseases and pathologies.
- The prevention of disease and extending the life of genetically disease-prone animals.
Physiological processes: Aging is accompanied by deterioration in many physiological reactions. Underfeeding is able to delay, blunt, or prevent most of these changes including age-related increases in serum cholesterol; decreasing the loss of dopamine receptors in the brain; slowing the age-related decline in the ability to learn a maze; prolonging the female reproductive function; preventing the decline in immune functions with age; inhibiting the decline with age of liver protein synthesis and proteolysis; and slowing the loss of locomotor activity.
Age-related diseases: In most studies, major age-associated diseases are the predominant cause of death. Food restriction has been shown to delay the onset and the severity of pathologies, or to totally prevent some pathologies of old age. Included are nephropathy, cardiomyopathy, hypertension-related lesions, neoplasia, diabetes, arthritis, and cardiovascular disease.
Disease-prone animals: Many animal species are predisposed to specific diseases, and laboratory animals have been selectively bred to study these disease processes. Generally, these animals develop diseases early in life and have a greatly reduced life span compared to normal strains of the same species. Underfeeding has the universal effect of delaying the early onset of pathology, reducing the incidence or severity of the pathology and extending the life span of disease-prone animals. In many instances, the increased life span exceeded the life span of free choice fed animals. These include animals genetically predisposed to the early onset of several types of autoimmune disease, cardiovascular disease, mammary adenocarcinoma, lymphoproliferative diseases, leukemia’s and lymphomas, gastric ulcers, cataracts, adenomas of the pituitary and pancreas, lung tumors, and genetically obese short lived animals.
Calorie Restriction: Mechanisms of Action
Instead of the previous question of, “How many calories should my dog eat?” The new questions is, “How does underfeeding increase the life span of canines?” This effect is due to a reduced level of energy intake, regardless of whether the energy comes predominantly from protein, carbohydrate, or fat. Early studies of McCay began food restriction shortly after weaning, and he postulated that food restriction extends the life span by slowing growth and delaying development. However, numerous studies have shown that calorie restriction beginning in young adulthood or even mid-life, were just as effective at prolonging life, delaying age-related changes in physiological processes and inhibiting pathologies.
- Reduction in oxidative free radical damage and an increase in antioxidant defense.
- Reduction in glycemia and plasma insulin.
Food restriction appears to protect animals against the damaging actions of reactive oxygen molecules. This protection involves a reduced rate of production of the molecules and an increased ability to scavenge them by elevation of antioxidant defense mechanisms. It should be said that underfeeding may also add to longevity by reducing the work load on all organ systems associated with digestion and the assimilation of food.
Antioxidant Protection
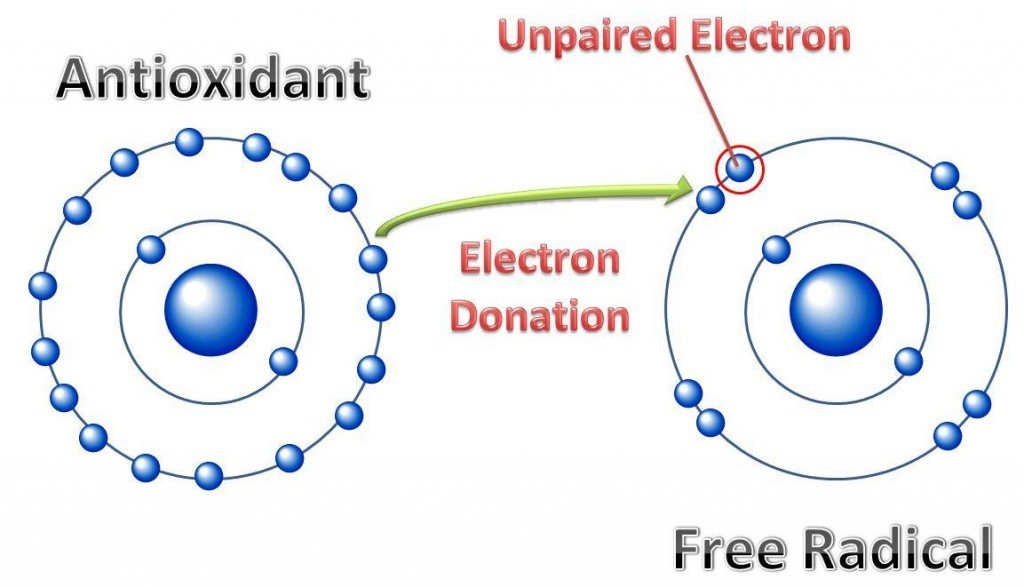
Free radical damage is responsible for the chronic age-related diseases that primarily cause the early demise of our pets. Free radicals are atoms that have lost an electron thus making them harmful when they come in contact with a healthy cell. Antioxidants neutralize free radicals by giving up one of their electrons to the free radical thereby protecting against potential cellular damage.
The emphasis on optimal compared to merely adequate nutrition has been most pronounced in the area of research concerning antioxidants. These nutrients include vitamin A, Beta-Carotene, vitamin C, vitamin E, and the minerals, selenium, zinc, manganese and copper.
Studies have shown that the antioxidant vitamins may be necessary at levels much higher than the Recommended Dietary Allowance to provide adequate protection against free radical damage. Furthermore, increased levels of antioxidants, either from dietary or supplemental sources appear to substantially reduce the risk of age-related diseases.
The Free Radical Theory of Aging was first proposed by Harman in 1972, and suggests that oxygen free radicals damage cells and tissues and the cumulative effect of this damage leads to aging, cell death and various age-related pathologies. All species that require oxygen for respiration have an elaborate antioxidant defense system to scavenge free radicals and protect cell DNA and other structures, particularly lipid membranes. Since oxygen free radicals are produced as a normal byproduct of energy metabolism, these destructive molecules must be constantly contained and neutralized.
The antioxidant defense system consists of antioxidant vitamins and antioxidant enzymes, some of which require essential minerals for activity.
| Nutritional Antioxidants | Enzyme Antioxidants |
|---|---|
| Vitamin A | Glutathione Peroxidase (selenium) |
| Beta Carotene | Superoxide Dismutase (manganese, copper, zinc) |
| Vitamin C | Glutathione Reductase |
| Vitamin E | Glutathione Transferase |
| Glutathione | Catalase |
This elaborate system functions intra-cellularly as well as extra-cellularly, and various components work synergistically. For example, when vitamin E interacts with a free radical, the vitamin E molecule becomes oxidized and cannot neutralize another free radical until it is again reduced. This reduction is generally accomplished by vitamin C, which in turn becomes oxidized. Vitamin C is restored to an active (reduced) form by glutathione, and the oxidized glutathione is fully recycled by the enzyme glutathione reductase to an active state. Without these synergistic interactions, the antioxidants would function one time and then be useless for further free radical scavenging. Obviously, a deficiency in one antioxidant can affect the entire defense system.
When animals are food restricted, they accumulate less lipofuscin and undergo less lipid peroxidation with age than ad libitum fed controls. Also, the antioxidant system that protects against this damage such as glutathione, glutathione reductase activity and catalase activity are increased by dietary restriction, compared to control aging animals that exhibit a decrease. Taken together, the studies strongly support the Free Radical Theory of Aging and suggest that underfeeding reduces the generation of reactive oxygen molecules and enhances the mechanisms, which protect cells from their damaging action.
Glucose and Insulin Control
Control of plasma glucose is dependent on insulin. Poor regulation of plasma glucose, as exhibited in insulin-dependent diabetes, results in serious complications including cardiovascular and renal disorders, neuropathy, cataracts, macular degeneration, aging of collagen and other tissue proteins, hypercholesterolemia, and declining immune response.
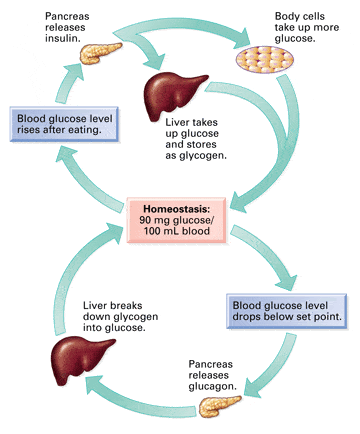
Food restricted animals were found to maintain mean plasma glucose levels significantly below ad libitum fed animals throughout their life span. In addition, underfed animals maintained plasma insulin at much lower concentrations than did ad libitum fed animals.
A recent study on rats supplemented with a highly bio-available form of chromium also supports this theory. Chromium is essential for insulin activity. In the study, rats were fed bio-available chromium during their lifetime, compared to control animals that received the same daily intake of inferior chromium generally found in most commercial feeds. Both groups were allowed to feed ad libitum. The animals receiving superior chromium had mean plasma glucose levels 35 mg/dl below control animals, while glycated protein and plasma insulin levels were less than one half the levels of controls. Finally, the superior chromium animals had a life span, which exceeded the control animals by 25%.
Effective glucose and insulin control is enhanced by food restriction. Therefore, the anti-aging effect of dietary restriction appears to be due to maintaining lower levels of plasma glucose and insulin throughout a life span without interfering with the use of glucose as fuel.
In summary, commercial pet food manufacturers, to date, have not utilized these long-standing nutritional advantages to any great extent. One can only hypothesize why the dog and cat have been denied optimal guidelines, ingredient quality, caloric management, antioxidant protection and the benefit of numerous nutrients that have been identified through research over the years.
Add years to your pet’s life and life to your pet’s years.












0 Comments